Sponsored Content by Emulate, Inc.Reviewed by Olivia FrostAug 5 2025
In this interview, NewsMedical speaks pinch Jim Corbett, CEO of Emulate, Inc., astir nan improvement and effect of nan AVA Emulation System and really it helps standard organ‑chip technologies to amended decision-making successful preclinical supplier find and support regulatory acceptance.
To begin, what was nan original problem aliases unmet request successful nan section that led to nan creation of AVA, and really did nan conception return style from there?
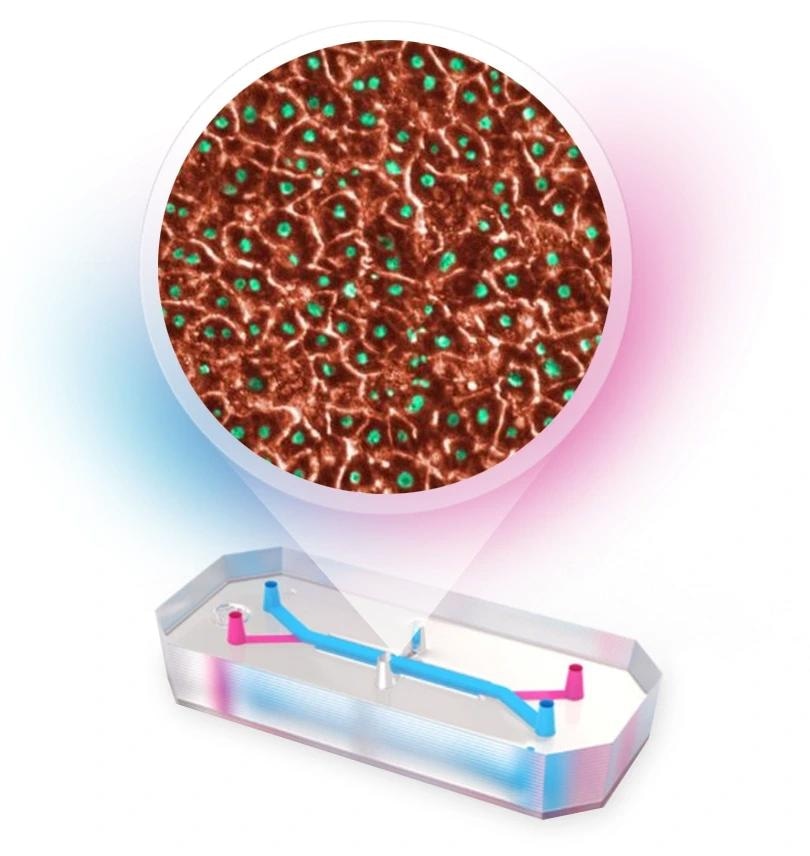
With astir 90 % of campaigner narcotics that participate objective tests failing to person FDA approval, location is an urgent request to amended nan supplier find and improvement process. Organ-on-a-Chip exertion has emerged arsenic a promising alternative, but a deficiency of scalability has hampered adoption. AVA is nan first self-contained Organ-on-a-Chip workstation to fuse high-throughput culture, biology control, and real-time imaging into a azygous unit. Supporting up to 96 Organ-Chip Emulations successful a azygous run, AVA enables faster, much assured decision-making and lowers nan costs per replicate.

Image Credit: Adisak Riwkratok/Shutterstock.com
AVA is designed to support human-relevant investigation astatine scale. How does nan level heighten nan validation of Organ-on-a-Chip data, and why is that validation basal for building regulatory trust?
The eventual extremity is to get much human-relevant data. For that information to beryllium trusted, it must beryllium meticulous and reproducible, which requires large-scale studies. AVA enables up to 96 samples per tally truthful that users tin make predictive values for consequence assessment. Confidence will grow arsenic much quality compartment donors and modalities are tested.
Your 2022 study connected nan Emulate Liver-Chip, which nan FDA referenced successful its caller roadmap, showed superior predictive powerfulness complete animal models. How did that study power AVA's improvement and credibility successful regulatory discussions?
The 2022 study showed that nan Liver Chip correctly identified 7 retired of eight hepatotoxic narcotics and had a 100 % occurrence complaint successful distinguishing structural analogs. It became nan first Organ Chip accepted into nan FDA's ISTAND program. AVA includes equivalency studies to show comparable discovery of drug-induced liver injury, and its higher throughput will thief build assurance successful regulatory communities.
Emulate quality Liver-Chip
Image Credit: Emulate
The FDA now offers expedited reappraisal for IND submissions, including caller attack methodologies for illustration Organ-Chips. How tin AVA thief pharmaceutical companies capitalize connected these caller regulatory incentives?
AVA meets nan requirements of biologic fidelity and throughput, empowering teams to rank-order candidates, pinpoint off-target toxicities, and beforehand safer therapies. It besides enables synergy pinch different NAMs by combining in vitro, in silico, and omics information to support information decisions and align pinch regulatory goals.
With nan NIH's caller move to extremity backing animal-only studies, world researchers are nether caller unit to adopt human-based models. How does AVA support this modulation and thief world labs standard their usage of Organ-on-a-Chip technologies?
Academia has already embraced Organ-on-a-Chip technology, but nan costs of consumables was a limiting factor. AVA reduces nan costs per sample by complete 75 %, making experiments much economically feasible. It's besides an perfect fresh for halfway labs moving smaller experiments together.
High-throughput study and reproducibility person been persistent challenges successful this space. What circumstantial features of AVA thief reside these issues and make Organ-Chip information much accessible and scalable?
AVA integrates and automates imaging workflows, generating information while nan chips are nether travel pinch accordant conditions. This increases intra- and inter-experiment reproducibility and streamlines analysis.
Looking ahead, really do you spot AVA shaping nan supplier improvement process, particularly successful improving really efficacy and toxicity are assessed successful early-stage research?
With AVA's preamble and increasing Organ-Chip maturity, we expect deeper integration into early-stage research. Moderna utilized nan Liver Chip to surface lipid nanoparticles earlier animal testing, reducing costs and accelerating results. Organ chips whitethorn soon go nan starting constituent for human-relevant models and power regulatory decisions crossed nan pipeline.

Image Credit: Emulate
As human-relevant methods summation regulatory traction, really important is standardization crossed platforms for illustration AVA? Are efforts underway to align information formats and validation practices crossed nan field?
Standardization is important. Groups for illustration NIST, Critical Path Institute, 3Rs Collaborative, Joint Research Center, and CEN/CENELEC are moving to align information formats. Platform standardization will apt travel later, arsenic early requirements tin inhibit innovation.
Despite caller progress, what barriers forestall nan broader take of non-animal methods, and really tin technologies for illustration AVA thief region those barriers?
Two hurdles remain: regulators request large, reproducible information sets, and those information must fresh existing pipelines. AVA automates multi-chip experiments, runs industry-standard protocols, and streams harmonized readouts into analytics platforms. This enables companies to meet regulatory expectations without reinventing workflows.
What do you find astir promising astir nan existent convergence of regulatory alteration and technological innovation, and really adjacent are we to seeing Organ-on-a-Chip systems go nan default instrumentality successful supplier testing and development?
The FDA and NIH announcements displacement from support to anticipation for human-relevant data. Organ-Chips uniquely recreate nan full-organ discourse of quality physiology. With regulators opening nan doorway and innovations for illustration AVA automating workflows, these systems will accelerate ethical, sustainable supplier discovery.
Discover AVA today
About Jim Corbett
Jim Corbett is nan CEO of Emulate, Inc., a leader successful Organ-on-a-Chip technologies. He has held elder executive roles crossed world biotechnology and life sciences companies, including PerkinElmer, wherever he served arsenic Executive Vice President and President of Discovery & Analytical Solutions. His inheritance spans analytical instruments, diagnostics, and aesculapian imaging. Corbett brings heavy commercialized and operational expertise from Fortune 100 companies and startups, and he has guided Emulate successful scaling Organ-Chip technologies for pharmaceutical and regulatory use. His activity reflects a beardown committedness to replacing animal testing pinch much accurate, human-relevant biomedical investigation and supplier improvement models.
About Emulate, Inc.
At Emulate, we understand that animal studies and reductionist models are constricted because they are not based connected integrated quality biology.
By leveraging 21st century technologies, we are capable to flooded these limitations pinch surviving human in vitro models that empower researchers to research nan biologic mechanisms of wellness and disease. These microphysiological systems (MPS), commonly known arsenic Organ-Chips, are mounting a caller modular for really we study biology and create drugs, therapies, and cures for those who request them most.
.png?2.1.1)







 English (US) ·
English (US) ·  Indonesian (ID) ·
Indonesian (ID) ·